- لا يوجد منتجات
* APIDRA is made by recombinant DNA (rDNA) technology in Escherichia coli.
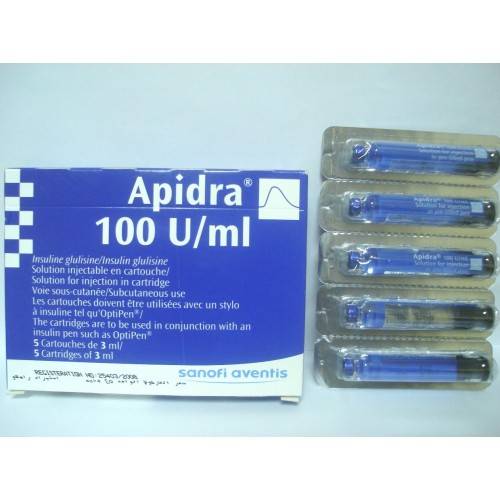
* Solution for injection in cartridge. The cartridges are to be used in conjuction with an insulin pen
such as OptiPen. Subcutaneous use.
* Use only clear and colourless solutions. Store in a refrigerator (2 – 8 oC), Do not freeze [in use conditions].
Once in use, cartridges may be kept for up to 4 weeks. Do not store above 25 oC and
keep in the outer carton in order to protect from light [Unopened]. Cartridges in use (in the insulin pen)
must not be stored in a refrigerator.
* Therapeutic indications:
Treatment of adult patients with diabetes mellitus.
* Posology and method of administration:
– APIDRA should be given shortly (0-15 minutes) before or soon after meals.
– APIDRA should be used in regimens that include an intermediate or long-acting insulin or
basal insulin analogue and can be used with oral hypoglycaemic agents.
– The dosage of APIDRA should be individualized adjusted.
# Administration:
– APIDRA should be given by subcutaneous injection or by continuous subcutaneous pump infusion.
– APIDRA should be administered subcutaneously in the abdominal wall, thigh or
deltoid or by continuous subcutaneous infusion in the abdominal wall. Injection sites and infusion sites
within an injection area (abdomen, thigh or deltoid) should be rotated from one injection to the next.
The rate of absorption, and consequently the onset and duration of action, may be affected by injection
site, exercise and other variables. Subcutaneous injection in the abdominal wall ensures a slightly
faster absorption than other injection sites.
– Care should be taken to ensure that a blood vessel has not been entered. After injection, the site of
injection should be massaged. Patients must be educated to use proper injection techniques.
# Mixing with insulins: Apidra must not be mixed with any preparations other than NPH (Neutral
Protamine Hagedorn) human insulin.
# Special populations:
– Renal Impairment: The pharmacokinetic properties of insulin glulisine are generally maintained in
patients with renal impairment. However, insulin requirements may be reduced the presence of
renal impairment.
– Hepatic Impairment: The pharmacokinetic properties of insulin glulisine have not been investigated
in patients with decreased liver function. In patients with hepatic Impairment, insulin requirements
may be diminished due to reduced capacity for gluconeogenesis and reduced insulin metabolism.
– Elderly: Limited pharmacokinetic data are available in elderly patients wih diabetes mellitus.
Deterioration of renal function may lead to a decrease in insulin requirements.
– Children and adolescents: There is no adequate clinical information on the use of Apidra in
children and adolescents.
* Contraindications:







المراجعات
لا توجد مراجعات بعد.