- لا يوجد منتجات
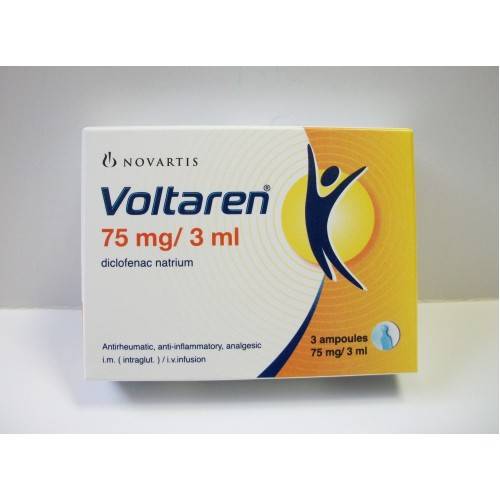
* Voltaren (diclofenac sodium) 75 mg/ 3 ml 6 ampoules. Antirheumatic, anti-inflammatory, analgesic. i.m. (intraglut.) / i.v. infusion.
* Composition: Each ampoule contains:
Sodium {o- {(2,6-dichlorophenyl) – amino} – phenyl} – acetate ( = diclofenac. natr.) 75 mg, excipients, preservative & solution for injection up to 3 ml.
* Therapeutic Indications:
# Intramuscular injection initial treatment of:
– Exacerbations of inflammatory and degenerative forms of rheumatism: rheumatoid arthritis, ankylosing spondylitis, osteoarthritis,
spondylarthritis, painful syndromes of the vertebral coulmn, non-articular rheumatism.
– Acute attacks of gout.
– Renal colic and biliary colic.
– Post-traumatic and postoperative pain, inflammation, and swelling.
– Severe migraine attacks.
# Intravenous infusion: Treatment or prevention of postoperative pain in a hospital setting.
* Dosage / administration:
– Adults: Voltaren ampoules should not be given for more than 2 days; if necessary, treatment can be continued with
Voltaren tablets or suppositories.
– Intramuscular Injection: The following directions for intramuscular injection must be followed in order to avoid
damage to a nerve or other tissue at the injection site. The dosage is generally one 75 mg ampoule daily, given by
deep intragluteal injection into the upper outer quadrant. In severe cases (e.g. colic) the daily dose can exceptionally
be increased to two injections of 75 mg, separated by an interval of a few hours (one into each buttock).
Alternatively, one ampoule of 75 mg can be combined with other dosage forms of Voltaren (tablets, suppositories)
up to a maximum daily dosage of 150 mg. In migraine attacks, clinical experience is limited to initial use of 1 ampoule of
75 mg administered as soon as possible, followed by suppositories up to 100 mg on the same day if required.
The total dosage should not exceed 175 mg on the first day. No data are available on the use of Voltaren to treat
migraine for more than one day. Should it be necessary to continue treatment on the following days, the maximum
daily dose is to be limited to 150 mg (given in divided doses in the form of suppositories).
– Intravenous infusion: Voltaren must not be given as an intravenous bolus injection. Immediately before starting an I.V.
infusion, voltaren must be diluted with saline 0.9% or glucose 5% infusion solution buffered with sodium bicarbonate.
Two alternative dosage regimens of voltaren are recommended: For the treatment of moderate to severe postoperative
pain, 75 mg should be infused continuously over a period of 30 minutes to 2 hours. If necessary, treatment may be
repeated after a few hours, but the dosage should not exceed 150 mg within any period of 24 hours. For the prevention
of postoperative pain, a loading dose of 25-50 mg should be infused after surgery over 15 minutes to 1 hour, followed by
a continuous infusion of about 5 mg per hour up to a maximum daily dosage of 150 mg.
– Children: Voltaren ampoules are contraindicated in children.
* Contraindications:
– Gastric or intestinal ulcer or bleeding.
– Known hypersensitivity to the active substance, or sodium metabisulphite and other excipients.
– Like other non-steroidal anti-inflammatory drugs (NSAIDs), Voltaren is also contraindicated in patients in whom attacks
of asthma, urticaria, or acute rhinitis are precipitated by acetylsalicylic acid or other drugs with prostaglandin-synthetase
inhibiting activity.
– Nasal polyps, angioedema, bronchospasm, or asthma.
– Moderate to severe impairment of renal function, hypovolaemia, or dehydration.
– Patients at high risk for postoperative bleeding or imcomplete haemostasis, haemotopoietic disorders, or cerebrovascular bleeding.
– Voltaren must not be used together with high doses of anticoagulants or with other anti-inflammatory agents.
– Voltaren ampoules must not be used in children, since there is no experience of such use.
* To be used under medical supervision.
* Protect from light and heat (store below 30 degrees C).
* Produced by: NOVARTIS PHARMA S.A.E. Cairo, under license from: Novartis Pharma AG, Basle, Switzerland.








المراجعات
لا توجد مراجعات بعد.