- لا يوجد منتجات
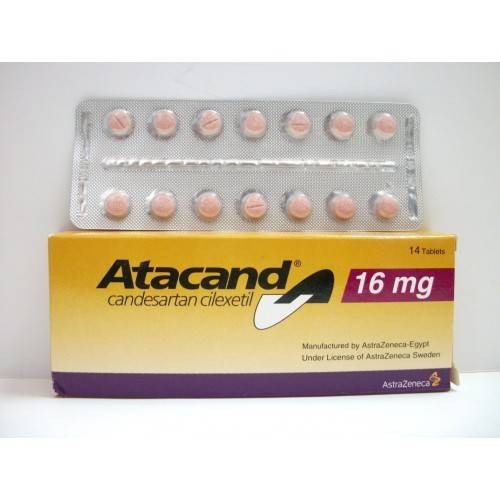
* Therapeutic Indications: – Essential hypertension.
& & – Treatment of patients with heart failure && impaired left ventricle systolic function (left ventricular ejection & & as add-on therapy to ACE inhibitors or when ACE inhibitors are not tolerated .
* Posology && Method of Administration:
& & # Dosage in Hypertension:
& & – The recommended initial && usual maintenance dose of Atacand is 8 mg once daily. In patients who require further
& & blood pressure reduction, a dose increase to 16 mg once daily is recommended. The maximal antihypertensive effect
& & is attained within 4 weeks of initiation of treatment.
& & In patients with less than optimal blood pressure reduction of Atacand, combination with a thiazide diuretic is recommended.
& & – Use in elderly: No initial dosage adjustment is necessary in eldely patients.
& & – Use in impaired renal function:
& & No initial dosage adjustment is necessary in patients with mild to moderate renal impairment (i.e. creatinine clearance >/=
& & 30 mL/min/1.73 m2 BSA). In patients with severe renal impairment (i.e. creatinine clearance <30 mL/min/1.73 m2 BSA,
& & the clinical experience is limited and a lower initial dose of 4 mg should be considered.
& & – Use in impaired hepatic function:
& & No initial dosage adjustment is necessary in patients with mild to moderate chronic liver disease. There is only limited
& & experience available in patients with severe hepatic impairment and/or cholestasis. A lower initial dose of 4 mg should
& & therefore be considered in these patients.
& & – Concomitant therapy: Addition of a thiazide-type diuretic such as hydrochlorothiazide has been shown to have an additive
& & antihypertensive effect with Atacand. (Atacand may be administered with other antihypertensive agents).
& & # Dosage in Heart Failure:
& & – The usual recommended initial dose of Atacand is 4 mg once daily. Up-titration to the target dose of 32 mg once daily or
& & the highest tolerated dose is done by doubling the dose at intervals of at least 2 weeks.
& & – Special patient populations: No initial dose adjustment is necessary for eldely patients or in patients with renal or hepatic
& & impairment.
& & – Concomitant therapy: Atacand can be administered with other heart failure treatment, including ACE inhibitors, beta-blockers,
& & diuretics && digitalis or a combination of these medicinal products.
& & # Administration: Atacand should be taken once daily with or without food.
& & # Use in children && adolescents: The safety && efficacy of Atacand have not been established in children && adolescents
& & under 18 years.
* Contraindications:
& & – Hypersensitivity to any component of Atacand.
& & – Pregnancy and lactation.
* Special warnings and precautions for use:
& & # Renal impairment:
& & – As with other agents inhibiting the renin-angiotensin-aldosterone system, changes in renal function may be anticipated
& & in susceptible patients treated with Atacand.
& & – When Atacand is used in hypertensive patients with renal impairment, periodic monitoring of serum potassium and
& & creatinine levels is recommended. There is limited experience in patients with very severe or end-stage renal impairment
& & (i.e. creatinine clearance<15 ml/min).
& & – Evaluation of patients with heart failure should include periodic assessments of renal function, especially in elderly patients
& & 75 years or older, and patients with impaired renal function. During dose titration of Atacand, monitoring of serum creatinine
& & and potassium is recommended. Clinical trials in heart failure did not include patients with serum creatinine >265 ?mol/L (>3 mg/dL).
& & # Concomitant therapy with an ACE inhibitor in heart failure:
& & The risk of adverse events, especially renal function impairment and hyperkalaemia, may increase when candesartan is
& & used in combination with an ACE inhibitor (see section 4.8 Undesirable effects). Patients with such treatment should be
& & monitored regularly and carefully.
& & # Renal artery stenosis:
& & Other drugs that affect the renin-angiotensin-aldosterone system, i.e. angiotensin converting enzyme (ACE) inhibitors,
& & may increase blood urea and serum creatinine in patients with bilateral renal artery stenosis or stenosis of the artery
& & to a solitary kidney. A similar effect may be anticipated with angiotensin II receptor antagonists.
& & # Kidney transplantation:
& & There is no experience regarding the administration of Atacand therapy in patients with a recent kidney transplantation.
& & # Hypotension:
& & Hypotension may occur during treatment with Atacand in heart failure patients. As described for other agents acting on
& & the renin-angiotensin-aldosterone system, it may also occur in hypertensive patients with intravascular volume depletion
& & such as those receiving high dose diuretics. Caution should be observed when initiating therapy and correction
& & of hypovolemia should be attempted.
& & # Anaesthesia and surgery:
& & Hypotension may occur during anaesthesia and surgery in patients treated with angiotensin II antagonists due to blockade
& & of the renin-angiotensin system. Very rarely, hypotension may be severe such that it may warrant the use of intravenous
& & fluids and/or vasopressors.
& & # Aortic and mitral valve stenosis (obstructive hypertrophic cardiomyopathy):
& & As with other vasodilators, special caution is indicated in patients suffering from haemodynamically relevant aortic or mitral
& & valve stenosis, or obstructive hypertrophic cardiomyopathy.
& & # Primary hyperaldosteronism:
& & Patients with primary hyperaldosteronism will not generally respond to antihypertensive drugs acting through inhibition
& & of the renin-angitensin-aldosterone system. Therefore, the use of Atacand is not recommended.
& & # Hyperkalaemia:
& & – Based on experience with the use of other medicinal products that affect the renin-angiotensin-aldosterone system,
& & concomitant use of Atacand with potassium-sparing diuretics, potassium supplements, salt substitutes containing potassium,







المراجعات
لا توجد مراجعات بعد.