- لا يوجد منتجات
-
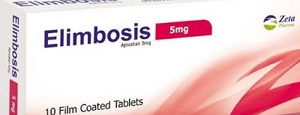
ELIMBOSIS 5 MG 30 TAB اليمبوسيس
EGP269.25
* Therapeutic indications
Brilique, co-administered with acetylsalicylic acid (ASA), is indicated for the prevention of atherothrombotic events in adult patients with:
– acute coronary syndromes (ACS) or
– a history of myocardial infarction (MI) and a high risk of developing an atherothrombotic event.
For at least the first 12 months following ACS, it is superior to clopidogrel.
* Pharmacotherapeutic group:
Platelet aggregation inhibitors excluding heparin, ATC code: B01AC24
* Mechanism of action:
– Brilique contains ticagrelor, a member of the chemical class cyclopentyltriazolopyrimidines (CPTP), which is an oral, direct acting, selective and reversibly binding P2Y12 receptor antagonist that prevents ADP- mediated P2Y12 dependent platelet activation and aggregation. Ticagrelor does not prevent ADP binding but when bound to the P2Y12 receptor prevents ADP-induced signal transduction. Since platelets participate in the initiation and/or evolution of thrombotic complications of atherosclerotic disease, inhibition of platelet function has been shown to reduce the risk of CV events such as death, MI or stroke.
– Ticagrelor also increases local endogenous adenosine levels by inhibiting the equilibrative nucleoside transporter-1 (ENT-1).
– Ticagrelor has been documented to augment the following adenosine-induced effects in healthy subjects and in patients with ACS: vasodilation (measured by coronary blood flow increases in healthy volunteers and ACS patients; headache), inhibition of platelet function (in human whole blood in vitro) and dyspnoea. However, a link between the observed increases in adenosine and clinical outcomes (e.g. morbidity-mortality) has not been clearly elucidated.





المراجعات
لا توجد مراجعات بعد.