- لا يوجد منتجات
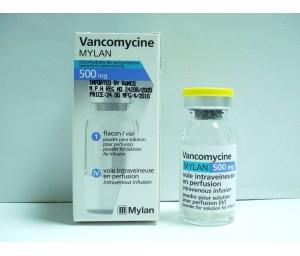
* Vancomycine MYLAN 500mg 1 vial of 500 mg, powder for solution for infusion (IV). intravenous infusion.
* Composition:
& & Vancomycin hydrochloride: 513.00 mg. Quantity corresponding to vancomycin base: 500.00 mg for one vial.
& & Powder for solution for infusion. Box of one vial, i.e. 500 mg of vancomycin base.
* Instructions: dissolve the content of one vial in 10 mL of water for injections.
& & TO BE DILUTED AFTER RECONSTITUTION OF THE SOLUTION (i.e. after the solution making).
* Method and route of administration: Strict intravenous use.
* Follow the prescribed dosages.
* Special storage conditions:
& & Do not store above 30 degrees C.
& & The reconstituted solution may be stored at 4 degrees C for 24 hours.
* List I – Medicinal product requiring hospital prescription.
* Marketing authorization holder: MYLAN S.A.S. – France.







المراجعات
لا توجد مراجعات بعد.