- لا يوجد منتجات
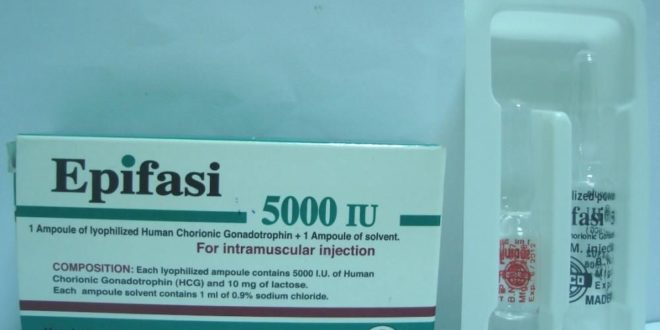
* Composition: – Each lyophilized ampoule contains:
& & 5000 I.U. of Human Chorionic Gonadotrophin (HCG) and 10 mg of lactose.
& & – Each ampoule solvent contains: 1 mL of 0.9% sodium chloride.
* Indications:
& & # Women:
& & – Anovulatory infertility due to absent or low concentrations of gonadotrophins; to promote
& & ovulation; where the cause of anovulation is not due to primary ovarian failure, and the
& & women have been appropriately pretreated with human menotrophins [Epigonal (R)].
& & – In combination with menotrophins as an adjunct to in-vitro fertilization procedures and
& & other assisted conception techniques.
& & # Men:
& & – To stimulate and maintain spermatogenesis in men with hypogonadotrophic hypogonadism.
* Dosage and Administration:
& & The solvent should be added to the lyophilized powder; the reconstituted solution of
& & Epifasi (R) should be immediately administered by intramuscular injection only. Any unused
& & portion should be discarded.
& & – Women:
& & Induction of ovulation and pregnancy: 5000 – 10000 units one day following the last dose of
& & menotrophins [Epigonal (R)].
& & – Men:
& & Infertility: 5000 I.U. is administered by intramuscular injection to stimulate spermatogenesis
& & 3 times/week for 4-6 months before administration of menotrophins [Epigonal (R)].
* Precautions:
& & – Combination therapy with Epigonal (R) for infertility should be used only by physicians
& & experienced with infertility problems.
& & – Induction of androgens secretion by Epifasi (R) may cause fluid retention; therefore the drug
& & should be used with caution in patients with:
& & . Epilepsy.
& & . Migraine.
& & . Asthma.
& & . Heart disease.
& & . Renal disorders.
& & – Patients suspected to be susceptible to hypersensitivity reactions should be given skin tests
& & before treatement.
* Pregnancy and Lactation:
& & – It is contraindicated during pregnancy (Category X).
& & – It should be used with caution during lactation.
* Contraindications:
& & – Hypersensitivity to the drug.
& & – Precocious puberty.
& & – Prostatic carcinoma or other androgen-dependent neoplasms.
& & – Breast, uterine, ovarian, and testicular tumors.
& & – Hypothalamus, pituitary, thyroid, and adrenal gland tumors.







المراجعات
لا توجد مراجعات بعد.