- لا يوجد منتجات
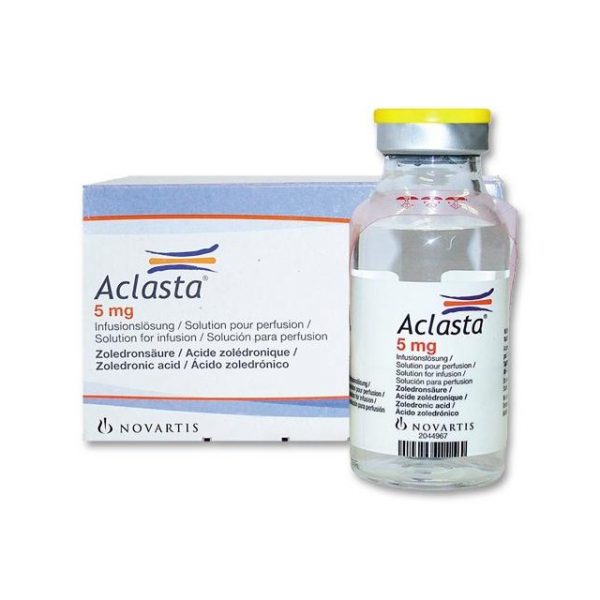
Aclasta (Zoledronic acid) 5 mg Solution for infusion. One bottle with 100 ml.
* Intravenous use.
* Indications and clinical usage:
– Postmenopausal osteoporosis: Aclasta (Zoledronic acid 5 mg / 100 mL) is indicated as a once-yearly intravenous infusion for the treatment of osteoporosis in postmenopausal women to reduce the incidence of hip, vertebral and non-vertebral fractures.
– Paget’s disease of the bone: Aclasta (Zoledronic acid 5 mg / 100 mL) is indicated as a single-dose intravenous infusion for the treatment of Paget’s disease of bone in men and women. Treatment is indicated in patients with Paget’s disease of bone with elevations in serum alkaline phosphatase (SAP) of at least two times the upper limit of age-specific normal reference range, or those who are asymptomatic, or those at risk for complications from their disease to induce remission (normalization of serum alkaline phosphatase). The effectiveness of Aclasta is based on serum alkaline phosphatase (SAP) levels.
– Geriatrics (>65 years of age): No overall differences in safety and efficacy were observed according to age.
– Pediatrics (<18 years of age): Safety and efficacy in children and growing adolescents have not been established. Aclasta should not be given to this patient population.
* Contraindications:
– Patients who are hypersensitive to this drug or to any ingredient in the formulation, or to any bisphosphonates or component of the container.
– Pregnancy and nursing mothers.
– Non-corrected hypocalcemia at the time of infusion.
* Warnings and precautions:
– Aclasta contains the same active ingredient that is found in Zometa (Zoledronic acid). Patients being treated with Zometa should not be treated with Aclasta.
– Patients being treated with Aclasta should not be treated with other bisphosphonates concomitantly.
– Infusion duration: The 5 mg single dose of Aclasta (Zoledronic acid 5 mg / 100 mL) should be infused in not less than 15 minutes.
* Pregnancy and lactation:
– Pregnant women: Aclasta should not be used during pregnancy as Zoledronic acid may cause fetal harm when administered to a pregnant woman.
– Nursing Women: It is not known whether Aclasta is excreted in human milk. Because many drugs are excreted in human milk, it should not be administered to a nursing woman.
* Dosage and Administration:
– Treatment of postmenopausal osteoporosis: The recommended dose is a once yearly single I.V. infusion of Aclasta.
– Treatment of Paget’s disease of bone: The recommended dose is a single I.V. infusion of Aclasta.
– Aclasta (5 mg in 100 mL ready to infuse solution) is administered intravenously via a vented infusion line.
– Patients should be advised to be appropriately hydrated before the administration of Aclasta.
– The infusion time must not be less than 15 minutes and the infusion rate should be constant. Aclasta should only be given by intravenous infusion. The total volume of Aclasta solution should be infused. Aclasta must never be given as a bolus injection.
* One bottle contains 5 mg zoledronic acid (anhydrous). Mannitol, sodium citrate and water for injections.
* Medicinal product subject to medical prescription. See package leaflet for handling.
* Do not store above 30 degrees C.
* Stable for 24 hours at 2 – 8 degrees C after opening.







المراجعات
لا توجد مراجعات بعد.